Research and Publication Ethics Committee (RPEC)
- About RPEC
- RPEC Committee
- Guidelines
- Application Procedure
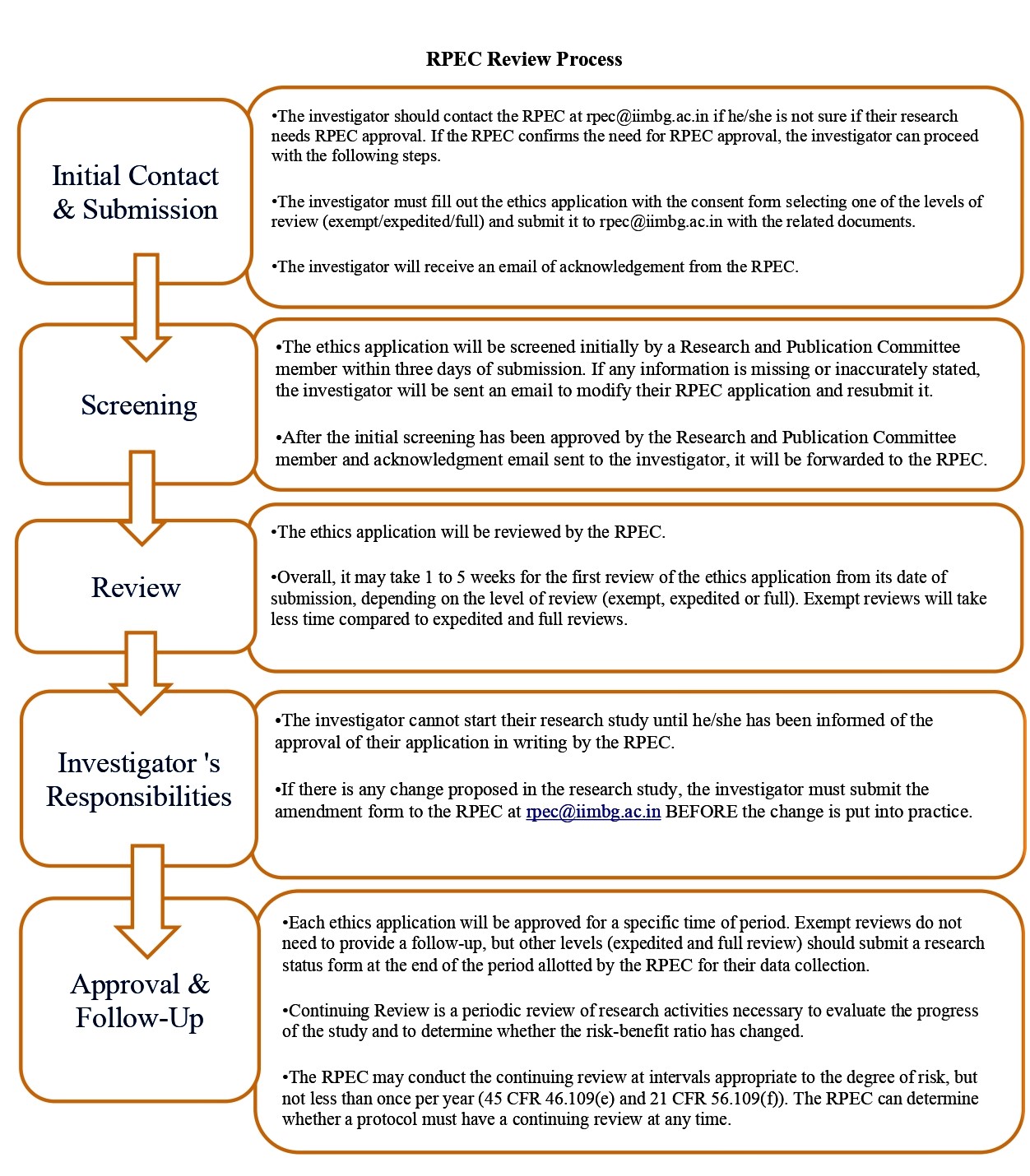
- Review Process
- Rules for recruiting subjects
- FAQs
About RPEC
The promotion of research culture among faculty members and students has been a significant activity undertaken by IIMBG over the past many years. IIMBG envisages a responsive role with a vision to promote intellectual contributions through academic research in all functional areas. In its endeavour to promote academic research and publication, IIMBG encourages faculty members to undertake externally funded major and minor research projects that can have an impact on society, business, and other stakeholders. IIMBG also provides seed money to the faculty members to undertake Minor/Major Research Projects (MRPs).
IIMBG is committed to perform an ethical review of research studies by protecting the rights and welfare of human subjects as well as supporting IIMBG’s research mission. The institute has a comprehensive review process to ensure that all research involving human elements addresses relevant ethical considerations and is subject to appropriate review.
Hence all research having human element considerations are referred to the Research and Publication Ethics Committee (RPEC), which has been constituted by the institute. The RPEC reviews all research involving human subjects before recruitment and data collection, which is conducted by IIMBG faculty, students, and staff. The proposals are reviewed in three cycles every year, i.e., in February, June, and October, or as and when required. RPEC is also known as the Institutional Ethics Committee (IEC), Institutional Review Board (IRB), Ethics Review Board (ERB), and Research Ethics Board (REB) in many countries and situations. Read More
To start a new application for a research study, please read the workflow and download the application form, and other related annexures from Application Procedure Tab. Submit duly filled form and related documents to rpec@iimbg.ac.in
The hard copy should be submitted to Member Secretary, RPEC.
Research and Publication Ethics Committee (RPEC)
The IIMBG Research and Publication Ethics Committee (RPEC) comprises of both internal and external members.
Internal Members: A senior faculty member from each area
External Members:
- Mr. Rakesh Kumar
Legal Expert - Dr. A.N. Tetarway
Psychiatrist / Psychologist / Medical expert
Ethics Application Form Click Here
- To start a new application for a research study, please download this ethics application form from the IIMBG website and fill in your responses and email to rpec@iimbg.ac.in with the related documents (See appendices in the ethics application form). Clear instructions have been provided in the form itself.
- Please submit the form and other documents in hard copy format to the Member Secretary. Submissions will be received on all working days.
- All the Research Proposals should be submitted to the Member Secretary at least 10 days before a scheduled Research and Publications Committee meeting. The meeting will be held at least once a three-month or as and when required. Those submitted later will be processed in the subsequent Research and Publications Committee meeting. Proposals receiving Ethical Clearance only shall be directed to the RPEC. Studies posing less than minimal risk to human participants shall be exempted from ethical clearance, and the clearance for that can be recommended by the Member Secretary to the Chairperson of RPEC based on the approvals of the Research and Publications Committee.
- No research project shall be started unless RPEC approval is obtained. Please bear in mind that no retrospective / post facto ethical clearance shall be provided to research projects which were neither submitted nor wetted by the RPEC
- If the approval is required in a particular format, a soft copy of such format may be submitted.
- All questions with an asterisk (*) are compulsory. Incomplete applications will be returned, and it will delay the review process.
- Level of review: You have to select one of the following options- excused, expedited, or full. Excused level should be selected if your interaction is benign (i.e., kind or gentle, not dangerous) and/or quite similar to everyday life events. Expedited level should be selected if the interactions are not necessarily benign and may induce discomfort physically or psychologically. Full review should be selected if the potential participants are going to be exposed to more than a minimal risk and these risks cannot be reduced through risk-minimizing procedures.
- For all questions with multiple options in this form, it has been clearly stated whether you need to pick one option or more.
- Guidelines for determining risk are provided in the application form on page 4. Please read carefully before filling in your response.
- You can add your scanned signature under Principal Investigator’s assurance on page 6.
- You can either fill out the editable version of the informed consent form template within this application OR have a new one with a similar format. The informed consent template is also available in an editable version on the IIMBG website. Please use the checklist for your informed consent form in either case.
- Please make sure you have attached files with all the research study material, including study stimuli, and video or audio recordings (if applicable).
- The research projects/proposal submitted should be as follows: Expand where necessary. Mention NA where not applicable.
Research Status Form
- This form is not for first-time applicants. They are for applicants whose RPEC application has been approved at least once by the RPEC.
- Please download these forms from IIMBG website and fill in your responses and email to rpec@iimbg.ac.in with the related documents mentioned in the forms.
Research Status Form Click Here
This form is needed post-approval of your ethics application by the RPEC. The RPEC will approve your research study for a certain period of time during which you can engage in data collection. After the expiration date of this time period, you should fill out this form. You will have three options in this form:
- Ongoing study/Extension request (Section A): If you need more time for data collection beyond the time approved by the RPEC, you should fill out this section.
- Completed study (Section B): If you have finished data collection successfully, you should fill out this section.
- Terminated study (Section C): If you decide to end the study without completing data collection for some unforeseen reason/s, you should fill out this section.
Research Amendment Form
- This form is not for first-time applicants. They are for applicants whose RPEC application has been approved at least once by the RPEC.
- Please download these forms from IIMBG website and fill in your responses and email to rpec@iimbg.ac.in with the related documents mentioned in the forms.
Research Amendment Form Click Here
This form is needed post-approval of your ethics application by the RPEC if you want to make any minor or major change in your research study. Examples of major and minor amendments are provided in the form itself. The RPEC has the discretion to decide whether or not a proposed amendment is major or substantial and requires ethical review.
If you have any questions, please send a message to rpec@iimbg.ac.in.- The RPEC Committee will require the submission of one original and one soft copy of each of the documents listed below for every research proposal.
- The documents required for submission are the following:
- RPEC Application form Click Here
- Research proposal
- Cover letter (Annexure 1) Click Here
- Resume of the Investigators (Annexure 2) Click Here
- Undertaking to follow ethical guidelines (Annexure 3) Click Here
- Undertaking that study hasn’t started yet (Annexure 4) Click Here
- Project risk assessment form (Annexure 5, should be submitted only if the risk involved is minimal or more than minimal) Click Here
- Tool translation undertaking (Annexure 6, should be submitted only if the local/regional language was used to collect data and then translated to English or vice-versa) Click Here
- Any study involving students, executive education participants, faculty, or full-time and contract staff at IIMBG as participants are to be conducted only after approval from the IIMBG RPEC.
- For research studies with Executive Education Program (EEP) participants, permission will be needed from the respective program Director/Coordinator every time. This permission is to be sought post-RPEC approval. The RPEC Chair should be informed of the program Director’s/Coordinator’s approval.
- For research studies with students from PGP and other degree-granting programs, the individual program chairs will be informed about the individual research studies post-approval from the RPEC. The RPEC will forward the details of the individual research studies to the individual chairs and Academic Offices (AOs) of the respective programs from where the researchers plan to recruit participants for their research. Along with the other ethical norms, the RPEC approval dealing with IIMBG students and EEP participants is subject to the following conditions:
- Cash incentives may be allowed for studies conducted outside the IIMBG campus for individuals not affiliated with IIMBG.
- Any cash or non-cash incentives for IIMBG/non-IIMBG participants should come from the researcher’s Faculty Development Allowance, Faculty Contribution Fund, or external sources (e.g., external grants).
- IIMBG RPEC strongly encourages studies with non-cash study design and non-cash incentives. However, if any researcher can provide substantial evidence stating that cash is a fundamental and non-negotiable part of the design of the research studies, the RPEC committee will consider it as a special case.
- If researchers from other institutions come to IIMBG for primary data collection, they are supposed to take permission from CAO (Chief Administrative Officer) and AO. If data collection is for secondary data (for which IIMBG is a subscriber), they should take permission from the Chairperson Research and Publications Committee (the application should be forwarded by the Area-Chair and by the Chief Librarian).
- Term paper/ summer projects or any other study conducted by the students as a part of their course or to fulfil the course requirement shall be exempted from taking RPEC clearance. If students are collecting data for their research work, which will be submitted to any journal or any agency or publisher to publish their research outcomes under the supervision of faculty members, where faculty members may or may not be authors, they are supposed to get RPEC clearance.
a. Any faculty member who is teaching a particular class should not conduct a research study (that is not part of the course) from the classes he/she is currently teaching to prevent any chance of coercion.
b. If the research study is part of the course (e.g., course project), it may be conducted with the teaching class with prior approvals from the RPEC. However, if the objective of the project/research study is to apply the techniques/concepts taught in the class, and one does not plan to publish or disseminate the results of the data, the project/research study may not require RPEC approval.
c. No cash incentive is allowed for any research study conducted on the IIMBG campus or off the IIMBG campus with IIMBG students, executive education participants, faculty, and full-time and contract staff.
d. Researchers should explain the rationale behind the selection of IIMBG students/community while applying for RPEC approvals.